Methionine is an essential amino acid associated with proper methylation supportive of the liver, brain, and DNA. It is one of only two sulfur-containing amino acids common among dietary proteins and essential to sulfur-dependent processes.
Methionine is dependent on several enzymes that are commonly noted to have mutations among healthy populations. The genes encoding for these enzymes, notably MTR and MTRR, may affect the methylation cycle in ways that could affect the detoxification of homocysteine, the methylation of DNA, and the production of antioxidants such as glutathione.
Table of Contents
Introduction
Methionine is considered an essential amino acid in that our bodies are not able to make it on their own. This means we must get an adequate amount via diet on a daily basis to avoid deficiency (1).
In addition to its essentiality, methionine is also a precursor to the conditionally essential amino acid Cysteine. It accomplishes this in combination with another amino acid, serine. Some probiotic compounds, namely Bacillus Subtilis (a soil-based organism) have been shown to aid in such conversions (3)
This means our bodies need help to produce these compounds during times of stress such as illness or injury. Methionine can therefore be considered an indirect precursor for other cysteine-dependent amino acids and compounds such as Taurine and Glutathione (2).
Biochemistry
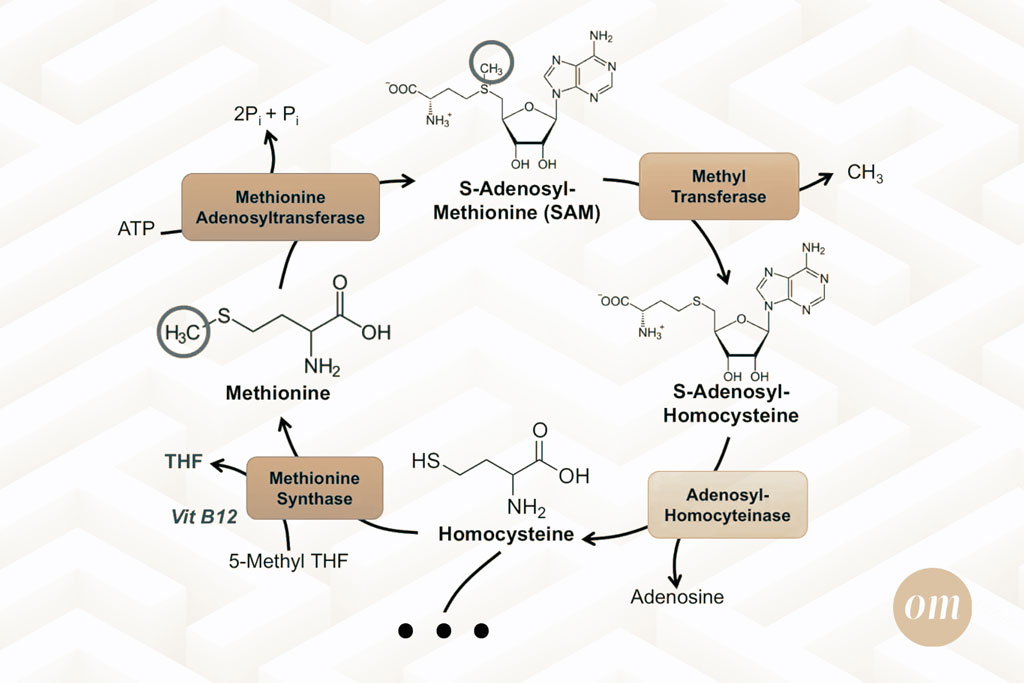
Our bodies use methionine to create SAMe which is essential to the production of neurotransmitters that affect mental health (10), maintaining healthy DNA structure (6), and even general liver health (9).
After being converted into SAM-e, methionine continues its journey until it becomes homocysteine. This compound, if left to accumulate in excess, has many known negative impacts on health ranging from miscarriages to autism spectrum disorder (11).
An enzyme named methionine synthase is responsible for the conversion of homocysteine back into methionine. This process requires the methyl-donor 5-methyl tetrahydrofolate (5-methyl THF) and B12 (12).
For more information on the Methionine Cycle pathway, I suggest watching this excellent video by JJ Science.
Genetic Factors Affecting Methionine Metabolism
Impairment in any process during the methionine cycle could conceivably affect methionine metabolism. There are, however, several stages during the cycle at which research has identified mutations occurring in higher frequency.
The enzyme 5-methyltetrahydrofolate-homocysteine methyltransferase (MTR) attaches a methyl group from methylcobalamin (b12) to homocysteine resulting in methionine. More information regarding MTR single nucleotide polymorphisms (SNPs) can be found here.
Another enzyme, 5-methyltetrahydrofolate-homocysteine methyltransferase reductase (MTRR) is tasked with the reactivation of the MTR enzyme. Mutations in genes affecting the production of this enzyme can have a significant impact on the function of the MTR enzyme and thus recycling of homocysteine back into methionine (13). More information regarding MTRR SNPs can be found here.
Recommended Daily Intake
The recommended daily intake for methionine and cysteine is approximately 1.3 grams per day for a 150-pound adult (19mg/kg) (1). These needs are increased during life stages such as pregnancy or during breast-feeding.
High Methionine Foods
Below is a table of some common foods with high relative amounts of methionine per 100g serving size. Note: the % RDA is calculated based on a 70KG person requiring 1,000mg/day.
| Food | Methionine (g/100g) | % RDA |
|---|---|---|
| Turkey | 931 | 84.64 |
| Beef | 905 | 82.27 |
| Tuna | 885 | 80.45 |
| Pork Chops | 850 | 77.27 |
| Cheese | 284 | 25.82 |
| Brazil Nuts | 1124 | 102.18 |
| Hemp Seeds | 933 | 84.82 |
| Lamb | 912 | 82.91 |
| Chicken | 859 | 78.09 |
| Anchovies | 855 | 77.73 |
| Ham | 779 | 70.82 |
Nutritional Considerations
Certain compounds have been shown to help mitigate deficiencies in methionine. Honokiol, an herbal extract from Willow bark, has been shown to help reduce the negative effects of methionine-deficient diets on the liver of mice (14). Obviously, there is not a definite translation between mice and humans but the results are certainly worth keeping in mind.
Research has shown that lower levels of serum methionine are associated with vegan diets (18), though not among less restricted vegetarian diets. Methionine levels were the highest among meat-eaters, fish eaters, and vegetarians. Supplementation may be required to maintain adequate levels of methionine among vegan diets.
Supplementation & Dosage
Methionine supplementation is sometimes used as a diagnostic tool to evaluate the cardiovascular risk associated with elevated blood homocysteine levels. These “loading doses” are reported as .1mg/kg of body weight — roughly 7 grams for a 150-pound adult (15).
Researchers have reported another single case where an accidental overdosing—a 100mg/kg dose—resulted in death. Older reports investigating methionine in relation to mental disease noted doses up to 10g per day not resulting in harm to patients (16).
Popular Methionine Supplements & Dosages
Below is a listing of dosages of popular methionine supplements from popular supplement brands. Note: some products, while offering a 500mg/capsule option, recommend a 2 capsule serving size for a total of 1,000mg serving size.
| Brand | Dose Per Capsule | |
|---|---|---|
| Solgar | 500mg | Link |
| Now Foods | 500mg | Link |
| Pure Encapsulations | 375mg | Link |
| Douglas Laboratories | 500mg | Link |
| Allergy Research Group | 500mg | Link |
| Nutricology | 500mg | Link |
| Blue Bonnet | 500mg | Link |
Health Benefits
The health benefits of methionine are broad, deeply-rooted, and intertwined with many complex processes. This is to be expected given methionine’s status as an essential amino acid—meaning it gets used for lots of things.
Below is a survey of several common aspects of human health in which supplementation or reduction in methionine has been studied as a potential therapeutic agent.
Arthritis
A study on rats indicated that a high methionine diet can help reduce the damaging effects of arthritis. This includes loss of skeletal mass in addition to improved appetite (4).
Bacterial-Related Inflammation
Lipopolysaccharide (LPS), the major component of the outer membrane of Gram-negative bacteria, is associated with damaging inflammation. Research shows supplementation capable of lowering inflammatory markers associated with LPS-originating inflammation (5).
In addition, the same researchers noted increased levels of cell-bound S-Adenosyl-L-Methionine (SAMe) which is also known to help lower markers of inflammation. Simply put; methionine therapy may help mitigate the damaging effects of bacterial infection and dysbiosis.
DNA Support
Methyl groups attach themselves to sequences in our DNA in ways that can alter how genes are expressed. This happens naturally in response to many biological processes and also in response to our environment (epigenetics) (6).
DNA methylation is facilitated by SAMe which, again, requires methionine to be available in adequate amounts. Methionine supports SAM-e levels by
SAM-e is a notoriously unstable compound, both in the body and as a supplement. Reports of initial SAM-e supplementation trials reported as much as 50% degradation during storage (8). Newer methods that involved individual packaging per tablet have been shown to help reduce this issue and provide more efficacious SAM-e.
Longevity
Methionine restriction has been shown to increase the lifespan in several limited animal studies. While research suggests methionine restriction may compare to the benefits shown from caloric restriction, these results have never been adequately replicated in human studies (17). More rigorous studies are needed to make this conclusion.
Acetaminophen Overdose Prevention
Methionine has been shown as effective as traditional treatments to treat liver damage associated with Acetaminophen overdoses (19). This therapy depends on methionine being administered within 10 hours of poisoning. Methionine offers the benefits of requiring a shorter treatment duration (12 hours vs. 3 days) and having no significant side effects.
Sulfur-Based Benefits
Methionine is one of only two sulfur-containing amino acids common to dietary proteins. Given that our bodies are unable to synthesize methionine, ensuring adequate dietary intake is essential to maintaining the supply of sulfur-based biochemical processes (20).
Common sulfur-based compounds of particular relevance to health are glutathione, taurine, cysteine, and all functions related to the transsulfuration pathway (21). This pathway, arguably more complex than even the methionine cycle illustrated earlier, is a primary driver of detoxification in the human body (22).
Grey Hair
A functional deficiency in methionine has been associated with the greying of a human hair (23). This research is only preliminary and makes no suggestion that methionine supplementation could effectively address grey hair. Nonetheless, it is certainly an interesting connection.
Final Thoughts
Methionine is, without doubt, essential to human health. Its sulfur-containing structure makes it one of few amino acids capable of supporting the broad range of sulfur-dependent pathways and processes in the body. The genetic alterations mentioned here could affect methionine status.
One of the most insightful DNA tests I had done was the StrateGene analysis which provides a detailed view of SNPs related to the methionine cycle and transsulfuration cycles. I would recommend this analysis to anyone looking for deeper insight into their genetics and methionine status.
References
- Institute of Medicine. 2005. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: The National Academies Press. doi: 10.17226/10490.
- Peter J. Reeds, Dispensable and Indispensable Amino Acids for Humans, The Journal of Nutrition, Volume 130, Issue 7, July 2000, Pages 1835S–1840S, doi: 10.1093/jn/130.7.1835S
- Hullo, Marie-Françoise et al. “Conversion of methionine to cysteine in Bacillus subtilis and its regulation.” Journal of bacteriology vol. 189,1 (2007): 187-97. doi:10.1128/JB.01273-06
- Mingxin Li, Lidong Zhai, Wanfu Wei, “High-Methionine Diet Attenuates Severity of Arthritis and Modulates IGF-I Related Gene Expressions in an Adjuvant Arthritis Rats Model“, Mediators of Inflammation, vol. 2016, Article ID 9280529, 6 pages, 2016, doi: 10.1155/2016/9280529
- Ji, Jian, et al. “Methionine Attenuates Lipopolysaccharide-Induced Inflammatory Responses Via DNA Methylation in Macrophages.” ACS Omega, vol. 4, no. 1, 2019, pp. 2331-2336, doi: 10.1021/acsomega.8b03571
- Moore, Lisa D et al. “DNA methylation and its basic function.” Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology vol. 38,1 (2013): 23-38. doi:10.1038/npp.2012.112
- Mischoulon, David, and Maurizio Fava. “Role of S-adenosyl-L-methionine in the treatment of depression: a review of the evidence.” The American journal of clinical nutrition vol. 76,5 (2002): 1158S-61S. doi:10.1093/ajcn/76/5.1158S
- Spillmann, M., Fava, M. S-Adenosylmethionine (Ademetionine) in Psychiatric Disorders. CNS Drugs 6, 416–425 (1996). doi: 10.2165/00023210-199606060-00002
- Guo, Tao et al. “S-adenosyl-L-methionine for the treatment of chronic liver disease: a systematic review and meta-analysis.” PloS one vol. 10,3 e0122124. 16 Mar. 2015, doi:10.1371/journal.pone.0122124
- Gao, J., Cahill, C.M., Huang, X. et al. S-Adenosyl Methionine and Transmethylation Pathways in Neuropsychiatric Diseases Throughout Life. Neurotherapeutics 15, 156–175 (2018). https://doi.org/10.1007/s13311-017-0593-0
- Azzini, Elena et al. “Homocysteine: Its Possible Emerging Role in At-Risk Population Groups.” International journal of molecular sciences vol. 21,4 1421. 20 Feb. 2020, doi:10.3390/ijms21041421
- Sanderson, S.M., Gao, X., Dai, Z. et al. Methionine metabolism in health and cancer: a nexus of diet and precision medicine. Nat Rev Cancer 19, 625–637 (2019). doi: 10.1038/s41568-019-0187-8
- van der Linden, I.J.M., den Heijer, M., Afman, L.A. et al. The methionine synthase reductase 66A>G polymorphism is a maternal risk factor for spina bifida. J Mol Med 84, 1047–1054 (2006). doi: 10.1007/s00109-006-0093-x
- Zhai, Ting et al. “Honokiol Alleviates Methionine-Choline Deficient Diet-Induced Hepatic Steatosis and Oxidative Stress in C57BL/6 Mice by Regulating CFLAR-JNK Pathway.” Oxidative medicine and cellular longevity vol. 2020 2313641. 27 Nov. 2020, doi:10.1155/2020/2313641
- Peter J. Garlick, Toxicity of Methionine in Humans, The Journal of Nutrition, Volume 136, Issue 6, June 2006, Pages 1722S–1725S, doi: 10.1093/jn/136.6.1722S
- Baldessarini, R J et al. “Methylation hypothesis.” Archives of general psychiatry vol. 36,3 (1979): 303-7. doi:10.1001/archpsyc.1979.01780030069006
- Lee, Byung Cheon et al. “Methionine restriction and life-span control.” Annals of the New York Academy of Sciences vol. 1363 (2016): 116-24. doi:10.1111/nyas.12973
- Schmidt, J., Rinaldi, S., Scalbert, A. et al. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: a cross-sectional analysis in the EPIC-Oxford cohort. Eur J Clin Nutr 70, 306–312 (2016). doi: 10.1038/ejcn.2015.144
- Vale, J A et al. “Treatment of acetaminophen poisoning. The use of oral methionine.” Archives of internal medicine vol. 141,3 Spec No (1981): 394-6. doi:10.1001/archinte.141.3.394
- Nimni, Marcel E et al. “Are we getting enough sulfur in our diet?.” Nutrition & metabolism vol. 4 24. 6 Nov. 2007, doi:10.1186/1743-7075-4-24
- Sbodio, Juan I et al. “Regulators of the transsulfuration pathway.” British journal of pharmacology vol. 176,4 (2019): 583-593. doi:10.1111/bph.14446
- Maclean, Kenneth N et al. “Cystathionine protects against endoplasmic reticulum stress-induced lipid accumulation, tissue injury, and apoptotic cell death.” The Journal of biological chemistry vol. 287,38 (2012): 31994-2005. doi:10.1074/jbc.M112.355172
- Wood, J M et al. “Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair.” FASEB journal : official publication of the Federation of American Societies for Experimental Biology vol. 23,7 (2009): 2065-75. doi:10.1096/fj.08-125435


